The U.S. Food and Drug Administration (FDA) has approved a device that serves as a new treatment option for moderate to severe sleep apnea. For the most part, the company, Respicardia is located out of Minnetonka, Minnesota.
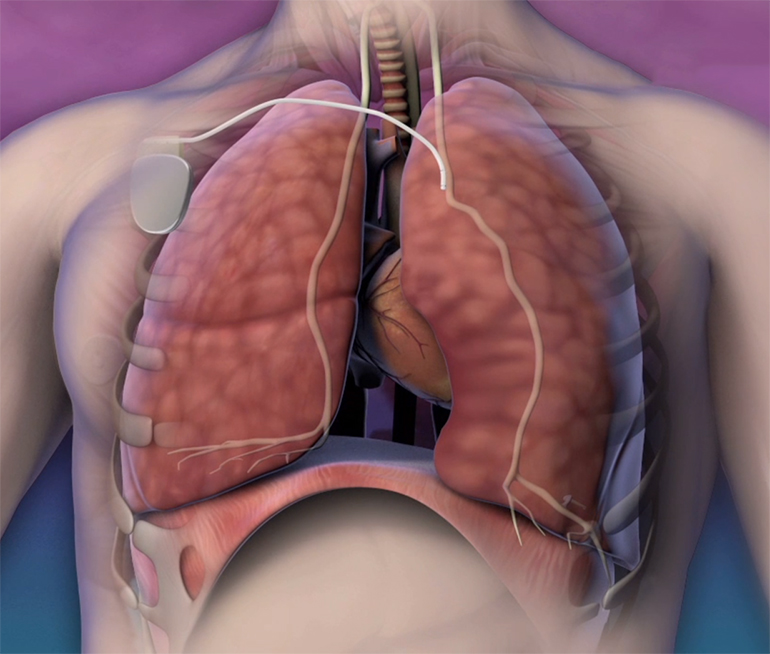
The company’s Remedē implantable sleep apnea treatment system works by stimulating a nerve in the chest that sends signals to the diaphragm telling it to move and induce breathing.
It’s important to note that sleep apnea is a condition that causes one or more pauses in respiration during sleep and it can last from a few seconds to minutes. This typically happens when the brain is unable to send signals from the phrenic nerve to the diaphragm to induce breathing. According to the National Institute of Health’s National Center on Sleep Disorders, this condition can lead to an increased risk of other health problems including heart attack, heart failure, high blood pressure, diabetes, obesity, and stroke.
The Remedē system consists of a battery pack that is surgically implanted under the skin as well as small wires that are inserted into the blood vessels near the phrenic nerve. The device monitors a patient’s breathing signals while they are sleeping, and if need be, stimulates the phrenic nerve to send signals to the diaphragm to restore normal respiration.
To test the effectiveness of the device in reducing sleep apnea, the FDA evaluated available data of 141 patients whose frequency and severity of their apnea events were monitored per hour using a measure known as the Apnea−Hypopnea Index (AHI). After six months, AHI was reduced by half in 50% of those with an active Remedē System implanted, compared to a reduction of just 11% in patients who didn’t have the device implanted.
Tina Kiang from the FDA’s Center for Devices and Radiological Health says, “patients with central sleep apnea should talk to their healthcare providers about the health risks and benefits of using this new treatment option over others, which include medication, the use of positive airway pressure devices and surgery.”
It’s not recommended to use the device to treat patients suffering from an active infection as well as those who typically need magnetic resonance imaging. Moreover, the Remedē System shouldn’t be used as a treatment option for obstructive sleep apnea.